By following these suggestions, pharmaceutical makers can make certain that their process validation actions meet the regulatory demands set forth with the FDA as well as the EMA.
By validating the process, organizations can have self esteem inside the regularity and reliability in their output techniques, leading to enhanced item excellent, elevated customer pleasure, and compliance with regulatory requirements.
Our Self esteem® authorities will conduct an E&L possibility assessment To judge the applicability from the extractable profile info (EU GMP Annex one) and guidebook you in the full validation process.
Process validation also contributes to continuous advancement attempts inside a company. By analyzing process info and figuring out spots for improvement, firms can increase their manufacturing processes, bringing about increased efficiency, diminished waste, and improved In general performance.
As per pointers, validation can be an act of demonstrating and documenting any Method, Process, and exercise that could systematically result in the anticipated success.
Get started eSigning pharmaceutical packaging validation protocol applying our Resource and sign up for the many contented customers who’ve already knowledgeable The important thing advantages of in-mail signing.
There isn't a improve while in the manufacturing process, plus the influence of improve from the manufacturing process will not be significant.
Use Skilled pre-developed templates to fill in and signal paperwork on the internet a lot quicker. Get usage of Many sorts.
hi there and welcome to my next executive series online video our subject matter is here process validation particularly protocols and studies aaron snyder below from top quality devices stated wherever we make quality techniques very simple hit the subscribe button to have all The great information we're earning check out the standing bar down below for the agenda and ensure you adhere all around for that reward questions our topic process validation protocols and stories will come straight from 820.seventy five and 1345 section seven.
Compliance with eSignature regulations is just a part of what airSlate SignNow can present to help make kind execution legal and secure. Moreover, it offers a lot of alternatives for sleek completion protection sensible.
Sartorius has long been a frontrunner in the sphere of extractables and leachables considering the fact that 1996, which means we carry deep knowledge of the science of extractables to every venture.
When the IQ has actually been carried out, the subsequent stage in process read more validation—operational qualification—makes certain that the machines is operating in accordance While using the person’s prerequisites and in the working array specified with the machine manufacturer. Quite simply, OQ makes confident the health-related unit is operating the best way it was intended to.
Implementing a systemwide possibility administration (SRM) method of manufacturing is critical to ensuring manufacturing tasks are vetted in an extensive and constant manner.
Process validation requires a number of actions occurring over the lifecycle on the product and process.
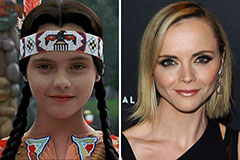 Christina Ricci Then & Now!
Christina Ricci Then & Now! Heath Ledger Then & Now!
Heath Ledger Then & Now! Danica McKellar Then & Now!
Danica McKellar Then & Now! Tiffany Trump Then & Now!
Tiffany Trump Then & Now! Bernadette Peters Then & Now!
Bernadette Peters Then & Now!